What is The Molarity of Pure Water? – Let’s Find Out
Here in this article we are going to explain What is The Molarity of Pure Water? Explore this scientific idea in depth with a user-friendly guide that makes it easy for everyone to understand the chemistry of pure water.
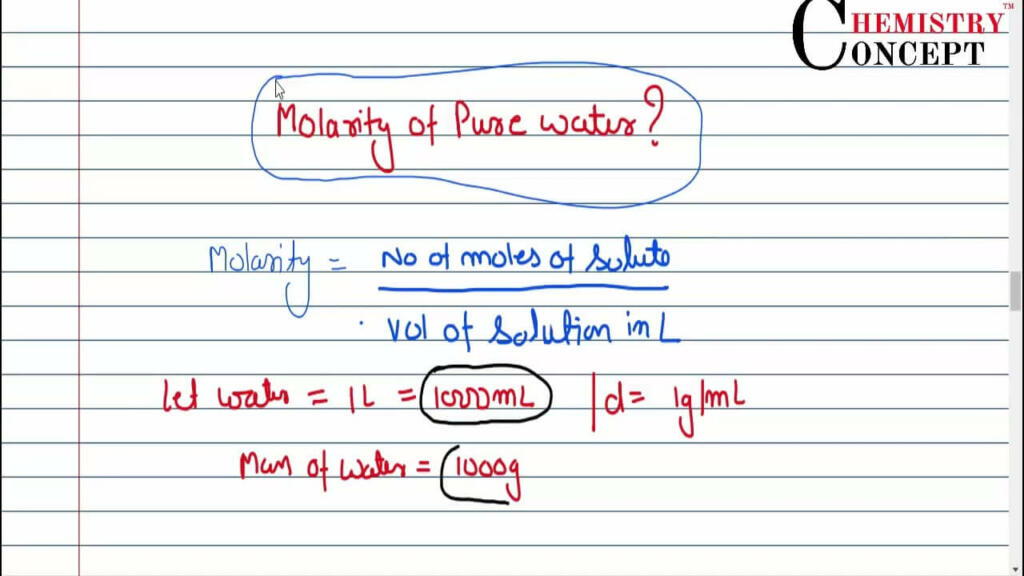
The Molarity of Pure Water Is:
As a result, the molarity of pure water is 55.56 mol/litre. Let’s have a non-scientific conversation about the molarity of pure water. Molarity is a fancy term used by scientists to describe the amount of a substance that is suspended in water. Now, though, what? Pure water is similar to an exclusive party that is only open to water molecules. No other guests are permitted.
Now, we typically count the number of friends (molecules) in a liter of water when discussing molarity. But it’s unique in the enchanted land of pure water. Pure water has a molarity of 55.56 mol/litre. How come? Because they are the only ones dancing on the dance floor, it’s like a party with only molecules of water.
Also Read: What is the Molar Mass of Phosphorus: Detailed Answer
Imagine this: If the water in this large tub is pure, it contains nothing but water—neither sugar nor salt—just water being its magnificent self. To put it in molarity terms, it indicates that there are zero moles of any other substance present in that liter of water.
To put it briefly, the molarity of pure water is equivalent to saying, “Hey, in this liter of water, there’s no other stuff – it’s just pure, cool water molecules having a good time on their own.” For this reason, the molarity of pure water is as simple as zero, meaning that additional guests are not needed for that H2O party!