India has emerged stronger from the storm of a once-in-a-century pandemic. The indigenous vaccines ensured that the country attain a healthy inoculation rate without being arm-twisted by the American Pharma-lobby. These indigenous vaccines have also trampled doomsday predictions prophesied by the Westerners and their sympathisers in India.
Amid all the gigantic health challenges, Indian scientists proved their scientific mettle and cemented India’s position at the top of the global vaccine manufacturers. These indigenous triumphed over the extensively advertised propaganda of ineffective, even lethal, foreign vaccines. With a startling discovery, Indian scientists have rewritten history. It will help in achieving the ambitious target of 100% inoculation and help fight any successive waves arising from any future mutation in the Coronavirus.
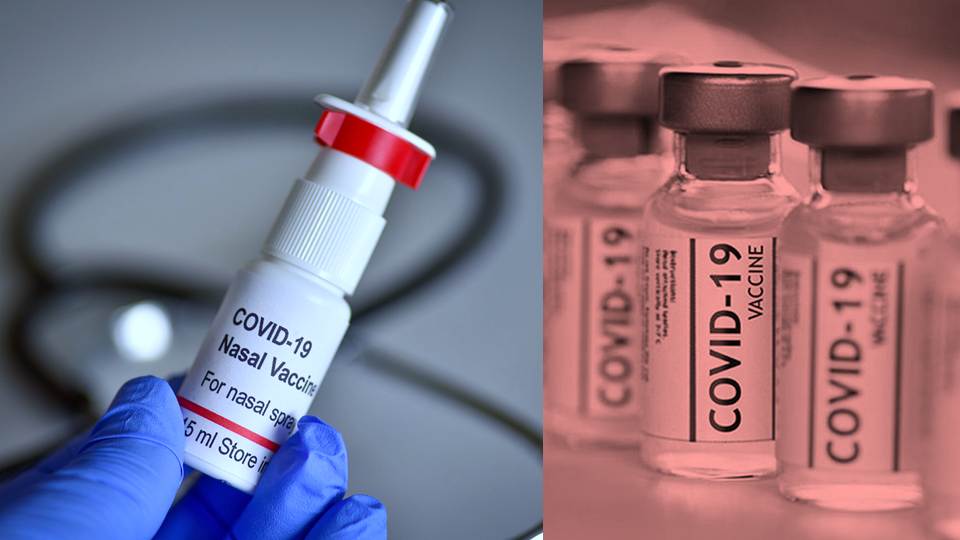
iNCOVACC: World’s first intra-nasal Covid vaccine
India has become the first country to approve an intranasal vaccine to combat the Covid virus. The Drugs Controller General of India (DCGI) has granted Bharat Biotech an emergency use authorisation (EUA) to for its intranasal vaccine, iNCOVACC. It is the first Covid vaccine that can be administered without pricking through a needle.
Bharat Biotech-made iNCOVACC becomes world's first intranasal COVID vaccine to get EUA
Read @ANI Story |https://t.co/gyyJBEFgLJ#BharatBiotech #iNCOVACC #IntranasalVaccine #COVID19 pic.twitter.com/1dfpeNClKM
— ANI Digital (@ani_digital) September 6, 2022
Also Read: India is all set to fight the menace of cervical cancer with its own indigenous vaccine
Mansukh Mandviya, Union Health Minister, tweeted about this significant development. The Minister lauded the country’s progress in Science, R&D and Human Resources. He emphasised that with science-driven approach and Sabka Prayas, we will defeat Covid-19.
This step will further strengthen our collective fight against the pandemic.
India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji's leadership.
With the science-driven approach & Sabka Prayas, we will defeat COVID-19.
— Dr Mansukh Mandaviya (मोदी का परिवार) (@mansukhmandviya) September 6, 2022
Also Read: India creates vaccine to keep animals around the world Covid-free
Bharat Biotech, the company behind iNCOVACC, released a statement on their scientific development. It read, “Bharat Biotech International Limited (BBIL), a global leader in vaccine innovation and developer of vaccines for infectious diseases, today announced that intranasal COVID vaccine (BBV154), has received approval under Restricted Use in Emergency Situation for ages 18 and above”.
iNCOVACC underwent safety tests ,and no unwarranted or major adverse events were noted during its trial. All the documented reactogenic and adverse events noticed during its safety trials were equivalent to those observed in other COVID-19 vaccines. Furthermore, the company stated that Product development data will be submitted to peer-reviewed journals and will be made available in the public domain.
Bharat Biotech has developed iNCOVACC, in partnership with Washington University in St. Louis. The American University designed and developed the recombinant adenoviral vectored constructs and evaluated them in preclinical studies for efficacy.
Product development at Bharat Biotech included preclinical safety review, large-scale manufacturing scale-up, formulation and delivery device development, and human clinical trials. The Government of India funded part of the product development and clinical trials under the Department of Biotechnology’s COVID Suraksha programme.
Bharat Biotech is all set to provide this nasal vaccine on a mass scale. For this purpose, it has established large manufacturing capabilities at multiple sites across India. These include manufacturing infrastructure at Gujarat, Karnataka, Maharashtra and Telangana.
What exactly is the iNCOVACC vaccine?
The iNCOVACC is a recombinant replication-deficient adenovirus vectored vaccine with a pre-fusion stabilised spike protein. As per Bharat Biotech, iNCOVACC intranasal vaccine is a heterologous booster dose. In heterologous boosting, a person can apparently be injected with a new vaccine regardless of which vaccine was used for the primary doses.
So, iNCOVACC can be administered as a booster dose to all citizens who have previously received 2 doses of the two commonly administered Covid vaccines in India.
The company statement said, “Immunogenicity was evaluated through serum neutralising antibodies by PRNT assays and serum IgG’s through ELISA’s. To evaluate vaccines taken through the intranasal route, IgA’s were evaluated by ELISA in serum and saliva. An evaluation was also carried out for the ability iNCOVACC to elicit long-term memory T and B cell responses against the ancestral and omicron variants”.
Also Read: India gets a standing ovation for its vaccine policy at Davos
Furthermore, iNCOVACC vaccinations are simple to store and distribute. Reportedly, Pfizer, which is not even very effective, requires higher logistical requirements. It must be stored at -70 Degrees C or its efficacy will be reduced. In comparison, iNCOVACC is stable at 2-8°C and can be easily maintained through normal refrigeration.
The vaccine candidate, iNCOVACC, performed well in all the three phases of clinical trials. The company has specifically formulated iNCOVACC vaccine so that it can be administered through nasal drops. This nasal delivery system is cost-effective and will be a game-changer in the ongoing fight against Covid pandemic in lower and middle-income countries.
How is it different from other COVID-19 vaccines?
Most of the Respiratory tract infections (RTIs) affect the nose, throat and lungs. These infections cause breathing problems which in several cases can be fatal. Patients infected with the Coronavirus experience similar symptoms and issues.
However, as per a scientific research, SARS-CoV-2, the coronavirus that causes COVID-19, infects the nasal cavity to a larger extent than in cells lower down the respiratory tract, including in the lungs. It replicates (increasing its population) aggressively in the nasal cavity as compared to lower respiratory tracts.
Also Read: From waiting for decades for vaccines to supplying 70% of global vaccines, India has come a long way
This is why this intranasal vaccine, iNCOVACC is different as it tackles the prime hotspot for Coronavirus infection.
Moreover, other vaccines require highly skilled manpower to administer it but this intranasal vaccine is easy to use and administer. Unlike other Covid vaccines, this one is easily accessible to households. It will be helpful in enabling mass immunisation to protect from emerging variants of concern. Since it can be administered through nasal drops, it completely eliminates the risk of injection induced side effects like swelling on springe spot, chills, muscle and joint pains.
All of these advantages will aid in immunising the hesitant population against Coronavirus while also eliminating the enormous infrastructure needs required for other COVID vaccines.
With the development of this new vaccine, India can ensure a 100% inoculation against Coronavirus and break the vicious cycle of mutations or variants of particular concerns. Furthermore, India has step-up as a responsible global power, assisting more than 150 countries in desperate need of vaccines, and these easy to use nasal vaccines will be India’s answer to the problems of lower and middle-income countries that are still badly gripped by Covid pandemic courtesy of greed of western nations.
Support TFI:
Support us to strengthen the ‘Right’ ideology of cultural nationalism by purchasing the best quality garments from TFI-STORE.COM
Also Watch: