As the second wave of the Coronavirus pandemic continues to rise towards an upward curve, the Indian government is planning to go into an overdrive mode by giving approvals to at least five vaccines by the end of the third quarter. News wire agency ANI quoting top government sources has revealed that Russia’s Sputnik V could be the first of the many vaccines that might be approved.
“India currently has 2 COVID-19 vaccines being manufactured locally: Covishield and Covaxin, and we can expect five more vaccines by Q3 2021. These vaccines are Sputnik V vaccine (in collaboration with Dr. Reddy’s), Johnson & Johnson vaccine (in collaboration with Biological E), Novavax vaccine (in collaboration with Serum India), Zydus Cadila’s vaccine, and Bharat Biotech’s Intranasal Vaccine. Safety and efficacy are the Union government’s primary concerns while granting emergency use authorisation (EUA) to any COVID-19 vaccine in the country,” the sources told ANI.
As per reports, Russia’s Sputnik V has an efficacy rate of 91.4 per cent and like the Covaxin and Covishield vaccine, it can also be stored at the fridge temperature, making it conducive for the Indian climate. The Russian vaccine is expected to be given the nod for rollout in the coming 10 days.
#COVID19 | Subject Expert Committee to meet today to take up Sputnik V application for Emergency Use Authorisation in India: Sources
— ANI (@ANI) April 12, 2021
Meanwhile, US pharma giant Johnson & Johnson, the only manufacturer with a single-dose Covid-19 vaccine, is expected to soon commence bridging clinical trials in the country. According to an Indian Express report, J&J has communicated its intention of making available the vaccines, at the earliest, to the government.
“Johnson & Johnson has sent a letter to the CDSCO (Central Drugs Standard Control Organisation) that they will very shortly apply for permission to conduct clinical bridging trials in India,”
Read More: Vaccine haters on Twitter, vaccines lovers in real life. The hypocrisy of opposition leaders
In large clinical trials, the J&J vaccine’s efficacy against the severe disease was 85.9 per cent in the United States, 81.7 per cent in South Africa, and 87.6 per cent in Brazil. In March, the World Health Organisation issued emergency use authorisation for the vaccine, paving the way for it to be used in the body’s vaccine distribution initiative.
Covovax, the vaccine jointly developed by the Serum Institute of India (SII) and Novavax has already shown positive signs in the early trials. SII’s CEO Adar Poonawalla had took to Twitter to announce the efficacy data.
“It has been tested against African and UK variants of Covid-19 and has overall efficacy of 89%. Hope to launch by September 2021,” Mr Poonawalla had tweeted.
Covovax trials finally begin in India; the vaccine is made through a partnership with @Novavax and @SerumInstIndia. It has been tested against African and UK variants of #COVID19 and has an overall efficacy of 89%. Hope to launch by September 2021! https://t.co/GyV6AQZWdV
— Adar Poonawalla (@adarpoonawalla) March 27, 2021
If rolled out, the vaccine will be the second from the company, the first being the Oxford-AstraZeneca vaccine produced locally in India.
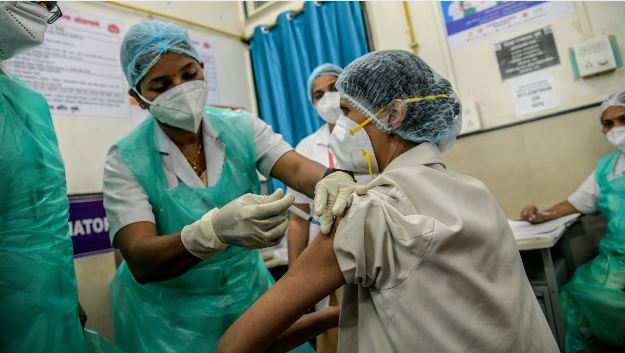
As the cases continue to rise across the country, the government has ramped up the vaccination drive considerably. India has overtaken the US in vaccinating the first 10 crores of the population. The Health Ministry claimed that India had administered these doses within 85 days, faster than the United States 89 days and China’s 103 days.
“In terms of the number of daily doses administered globally, India continues to remain at the top with an average of 38,34,574 doses administered per day,” the ministry said.
India had set an all-time record of administering 45 lakhs doses on April 5 which is expected to be breached in the coming days after the vaccine production process is expedited with the introduction of newer vaccines.